After fertilization, embryos race through rapid cell divisions before slowing down to build specialized cells that will carry out distinct functions in the developing body—but the signals that trigger this shift have remained a mystery.
In a new study published in EMBO Reports, Dartmouth biologists working with fruit flies shed light on how embryos sense when it’s time to reorganize their DNA and activate their own genes. This work opens the door to understanding how similar mechanisms might operate in humans.
“The way that the DNA is packaged changes pretty dramatically in the very early embryo, as it goes from maternal control to transcribing its own genes,” says senior author Amanda Amodeo, an assistant professor of biological sciences. “We showed that this change is related to the embryo’s nuclear-to-cytoplasmic ratio, which could have implications for understanding certain cancers and aging in humans, which are marked by disruptions in this ratio.”

Biological sciences professor Amanda Amodeo and Guarini PhD student Anusha Bhatt
Fruit flies are a favorite model for studying embryonic development because researchers have extensive genetic tools for them, and their embryos develop inside a transparent shell—perfect for imaging under a microscope. These tiny eggs, about the size of a needle tip, hatch within 24 hours.
“Drosophila has, time and time again, helped researchers identify fundamental biological processes that end up being important for human and animal well-being, and we hope that’s also the case here,” says Amodeo.
Early embryo development is partially preprogrammed by proteins the embryo inherits from its mother. These maternal proteins are present in the egg prior to fertilization and help govern processes within the embryo before it has time to produce its own proteins.
Fruit fly embryos take the reins and begin controlling their own development after 13 rounds of rapid, synchronized cell division. At this point, the same space that originally held a single nucleus now contains over 6,000 nuclei.
Amodeo thought that proteins called histones might play a role in coordinating the shift from maternal to embryonic control in fruit flies and other animals.
DNA wraps around histone protein complexes like beads on a string, keeping it organized, untangled, and protected from damage. Previous research has shown that specific histones are associated with gene activation transcription in adult cells, but their role in embryonic development was unknown.
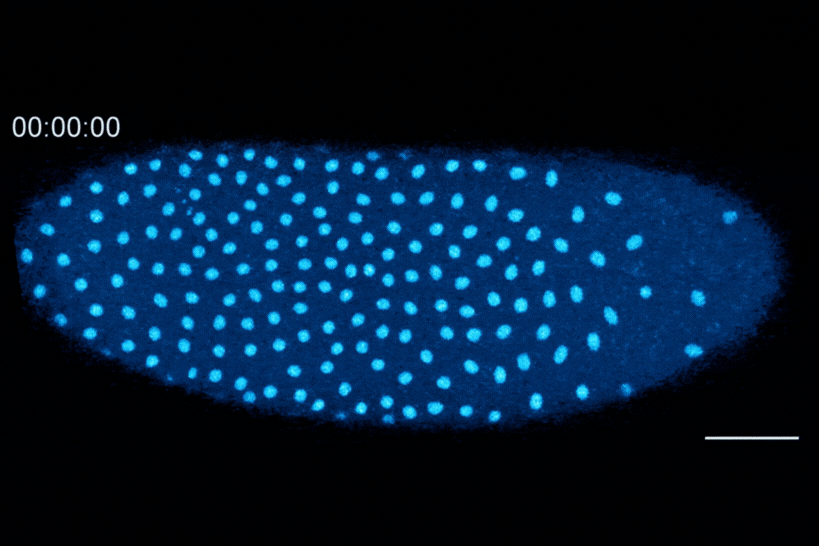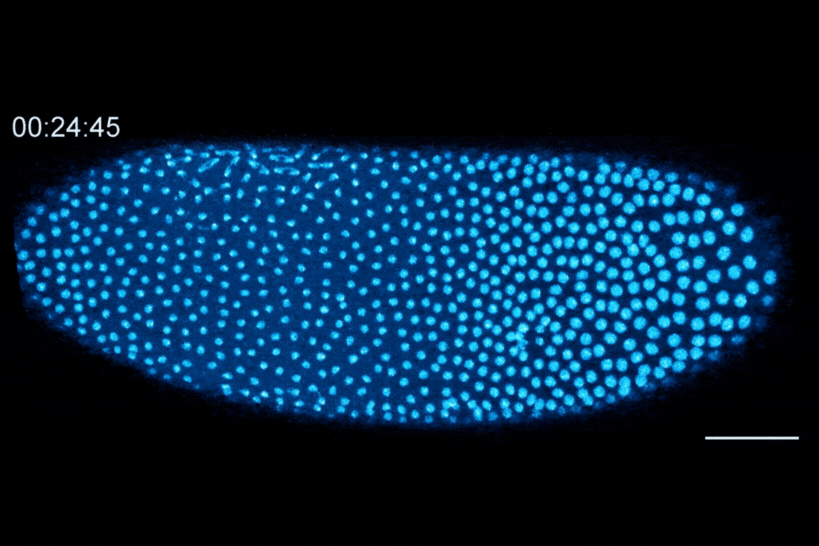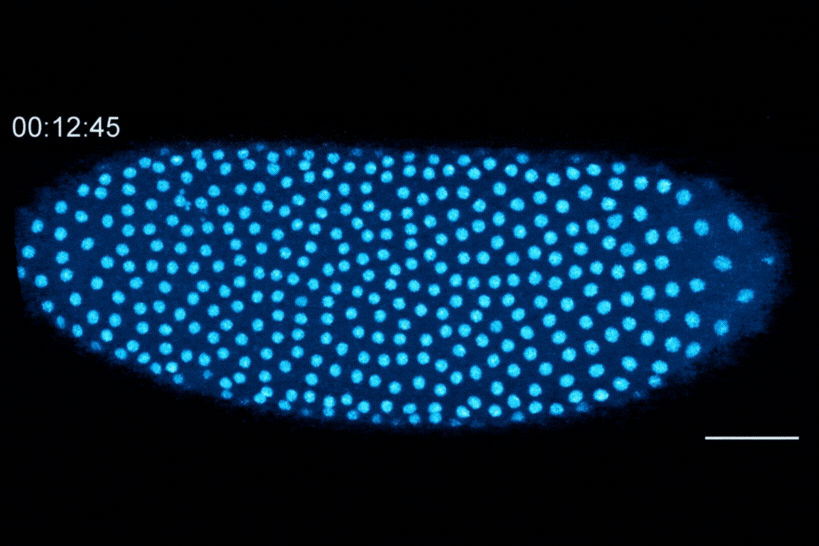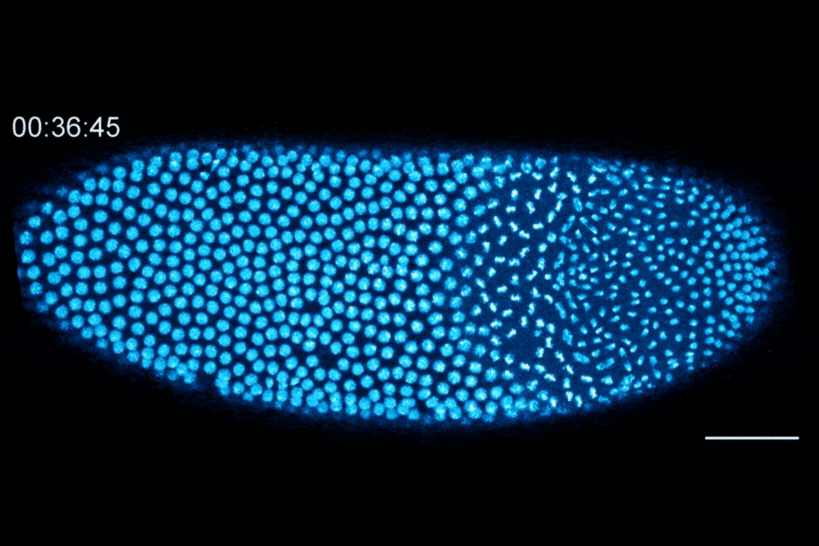
The Dartmouth team showed that cell division in early fruit fly embryos is tied to a histone called H3.3, which replaces histone H3 when the embryo senses that it contains a high concentration of nuclei. This fruit fly embryo has a mutation that prevents its nuclei (shown in blue) from spreading out evenly, resulting in regions with different nuclear-to-cytoplasmic ratios. This results in asynchronous waves of nuclear division, where the dense regions divide more slowly than the less dense regions.
Amodeo previously showed that when embryos sense that their DNA has reached a critical threshold after repeated doubling, they swap a histone known as H3 for a variant called H3.3. Since H3.3 is associated with gene activation, the researchers think this repackaging may contribute to embryos moving onto their next phase of development.
“Even though they only differ by four amino acids, H3 and H3.3 have very different functions,” says Anusha Bhatt, a Guarini PhD student in Amodeo’s lab and first author of the paper. “H3 is your go-to histone when you just want to make a copy of your genome. But H3.3 is used in specific regions within the genome where you want to transcribe a lot of genes.”
The researchers began investigating whether histones are involved in early embryo development by first tracking how the amount of H3 and H3.3 changes in the nuclei of fruit fly embryos as they approach the 13th round of cell division. Using fluorescently tagged histones, they showed that the amount of H3 associated with the embryo’s DNA decreases with each cell division, while the amount of H3.3 increases.
The researchers found that the switch from H3 to H3.3 is triggered when embryos sense that they have become crowded with nuclei. In embryos that lacked the ability to distribute their nuclei evenly, regions that were more crowded with nuclei showed higher levels of H3.3 incorporation, whereas uncrowded regions did not make the shift to replace H3 with H3.3.
For future studies, the team plans to further investigate the role of H3.3 in genome activation. “I want to know where exactly in the genome H3.3 is being incorporated during those initial cycles, and what genes it’s activating,” says Bhatt.
Two Dartmouth undergraduate students are coauthors on the paper—Madeleine Brown ’22, who is now a PhD student at Cornell University, and Aurora Wackford ’26.
“Anusha is the first graduate student to join my lab at Dartmouth, and Maddie was one of the first undergraduates to join my lab, so I'm very proud of both of them and this work,” says Amodeo.
